5.5 Haemostasis
Learning Objectives
By the end of this section, you will be able to:
- Describe the three mechanisms involved in haemostasis
- Explain how the extrinsic and intrinsic coagulation pathways lead to the common pathway and the coagulation factors involved in each
- Discuss disorders affecting haemostasis
Platelets are key players in haemostasis, the process by which the body seals a ruptured blood vessel and prevents further loss of blood. Although rupture of larger vessels usually requires medical intervention, haemostasis is quite effective in dealing with small, simple wounds and small internal bleeds. There are three steps to the process: vascular spasm, the formation of a platelet plug, and coagulation (blood clotting). Failure of any of these steps will result in haemorrhage—excessive bleeding. The vascular response and platelet plug formation are referred to as primary haemostasis and coagulation is referred to a secondary haemostasis.
Vascular Spasm
When a blood vessel is severed or punctured, or when the wall of a vessel is damaged, vascular spasm occurs. In vascular spasm, the smooth muscle in the walls of the vessel contracts dramatically. This smooth muscle has both circular layers; larger vessels also have longitudinal layers. The circular layers tend to constrict, which narrows the vessel lumen, and slows the flow of blood. The longitudinal layers, when present, draw the vessel back into the surrounding tissue, often making it more difficult for a surgeon to locate, clamp, and tie off a severed vessel. The vascular spasm response is believed to be triggered by several chemicals called endothelins that are released by vessel-lining (endothelial) cells and by pain receptors in response to vessel injury. This phenomenon typically lasts for up to 30 minutes, although it can last for hours.
Formation of the Platelet Plug
In the second step, platelets, which normally float free in the plasma, encounter exposed underlying connective tissue and collagenous fibres at the site of blood vessel damage. The platelets bind (adhesion) to the exposed collagen and become activated. Activated platelets are spiked and sticky and bind to other activated platelets (aggregation) and the endothelial lining. Platelet adhesion is assisted by a glycoprotein released from neighbouring endothelial cells called von Willebrand factor, which helps stabilise the growing platelet plug. Platelet aggregation is aided by the plasma protein, fibrinogen, which forms bridges between adjacent platelets at the site of bleed vessel damage. As platelets collect, they simultaneously release chemicals from their granules into the plasma that further contribute to haemostasis. Among the substances released by the platelets are:
- adenosine diphosphate (ADP), which helps additional platelets to adhere to the injury site, reinforcing and expanding the platelet plug
- serotonin, which maintains vasoconstriction
- prostaglandins and phospholipids, which also maintain vasoconstriction and help to activate further clotting chemicals, as discussed next
- Von Willebrand factor which aids in the adhesion of platelets to exposed collagen
A platelet plug can temporarily seal a small opening in a blood vessel. Plug formation, in essence, buys the body time while more sophisticated and durable repairs are being made. In a similar manner, even modern naval warships still carry an assortment of wooden plugs to temporarily repair small breaches in their hulls until permanent repairs can be made.
Coagulation
Those more sophisticated and more durable repairs are collectively called coagulation, the formation of a blood clot. The process is sometimes characterised as a cascade, because one event prompts the next as in a multi-level waterfall. The result is the production of a gelatinous but robust clot made up of a mesh of fibrin—an insoluble filamentous protein derived from fibrinogen, the plasma protein introduced earlier—in which platelets and blood cells are trapped. Figure 5.5.1 summarises the three steps of haemostasis.
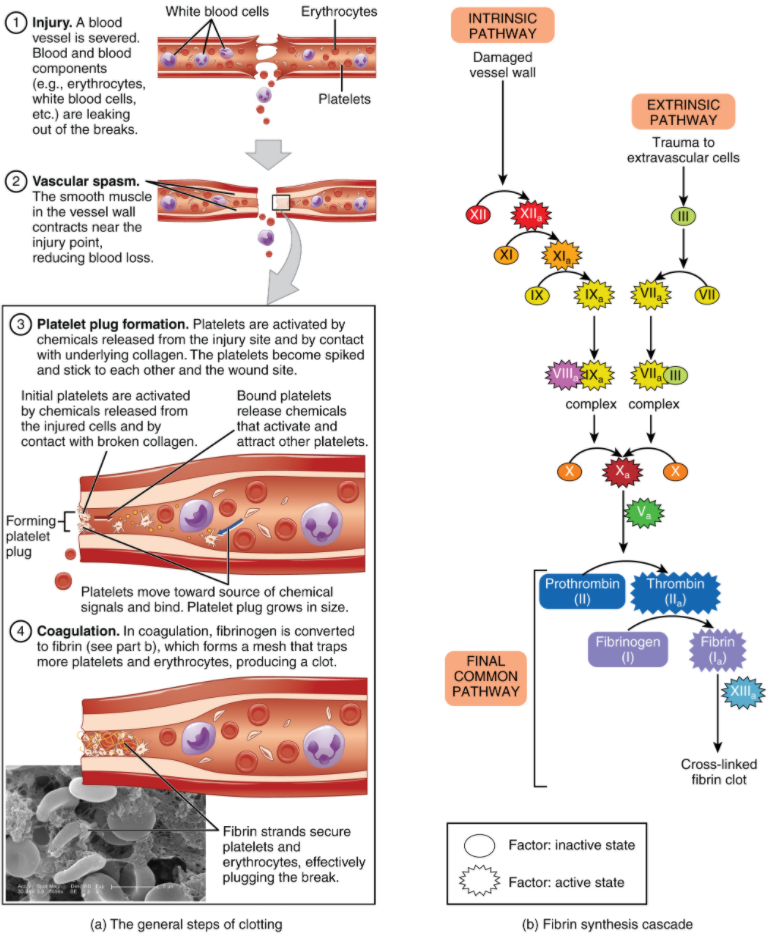
Clotting Factors Involved in Coagulation
In the coagulation cascade, plasma proteins called clotting factors (or coagulation factors) prompt reactions that activate still more coagulation factors. The process is complex, but is initiated along two basic pathways:
- The extrinsic pathway, which is triggered when clotting factors come into contact with substances outside of the blood vessel.
- The intrinsic pathway, which is triggered when clotting factors come into contact with substances inside the blood vessel.
Both of these pathways merge into a third pathway, referred to as the common pathway (see Figure 5.5.1b). All three pathways are dependent upon the 12 known clotting factors, including Ca2+ and vitamin K (Table 5.5.1). Clotting factors are secreted as inactive enzymes primarily by the liver and the platelets. The liver requires the fat-soluble vitamin K to produce many of them. Vitamin K (along with biotin and folate) is somewhat unusual among vitamins in that it is not only consumed in the diet but is also synthesised by bacteria residing in the large intestine. The calcium ion, considered factor IV, is derived from the diet and from the breakdown of bone. Some recent evidence indicates that activation of various clotting factors occurs on specific receptor sites on the surfaces of activated platelets.
The 12 clotting factors are numbered I through XIII according to the order of their discovery. Factor VI was once believed to be a distinct clotting factor, but is now thought to be identical to factor V. Rather than renumber the other factors, factor VI was allowed to remain as a placeholder and also a reminder that knowledge changes over time. Activated factors are denoted by the additions of the letter “a” after the factor number. For example, factor Va denotes activated factor five.
Table 5.5.1. Clotting factors
*Vitamin K Required
[table “5” not found /]
Extrinsic Pathway
The quicker responding and more direct extrinsic pathway (also known as the tissue factor pathway) begins when damage occurs to the surrounding tissues, such as in a traumatic injury. Damaged extravascular cells, which are extrinsic to the bloodstream, express factor III, also known as tissue factor or thromboplastin. When factor VII in the plasma comes into contact with exposed factor III, it becomes activated to factor VIIa. Factor VIII, Factor VIIa and Ca2+ then form an active enzyme complex. This enzyme complex leads to activation of factor X (Stuart–Prower Factor), which activates the common pathway discussed below. The events in the extrinsic pathway are completed in a matter of seconds.
Intrinsic Pathway
The intrinsic pathway (also known as the contact activation pathway) is longer and more complex. In this case, the factors involved are intrinsic to (present within) the bloodstream. The pathway can be prompted by damage to the tissues, resulting from internal factors such as arterial disease; however, it is most often initiated when factor XII (Hageman factor) comes into contact with negatively charged molecules, such as phospholipids expressed on the surface of activated platelets. Factor XII can also be activated by contact with foreign materials such as artificial heart valves and the surface of some bacteria. Factor XII is also activated when blood comes into contact with glass, in a test tube for example. Factor XIIa sets off a series of reactions that in turn activates factor XI (plasma thromboplastin antecedent) then factor IX (antihemophilic factor B or plasma thromboplasmin). In the meantime, substances released by the platelets increase the rate of these activation reactions. Finally, factor IXa binds to its cofactor, factor VIII (antihemophilic factor A) to form an enzyme complex that activates factor X (Stuart-Prower Factor or thrombokinase), leading to the common pathway. The events in the intrinsic pathway are completed in a few minutes.
Common Pathway
Both the intrinsic and extrinsic pathways lead to the common pathway, in which fibrin is produced to seal off the vessel. Once factor X has been activated to become the enzyme prothrombinase (factor Xa) by either the intrinsic or extrinsic pathway, it converts factor II, the inactive enzyme prothrombin, into the active enzyme thrombin. (Note that if the enzyme thrombin were not normally in an inactive form, clots would form spontaneously, a condition not consistent with life.) Then, thrombin converts factor I, the soluble plasma protein fibrinogen, into the insoluble protein fibrin protein strands. Fibrin molecules spontaneously join together to form a mesh, which is stabilised by cross-linking reactions catalysed by Factor XIIIa.
Fibrinolysis
The stabilised clot undergoes contraction via the action of contractile proteins within platelets. As these proteins contract, they pull on the fibrin threads, bringing the edges of the clot more tightly together, somewhat as we do when tightening loose shoelaces. This process also wrings out of the clot a small amount of fluid called serum, which is blood plasma without its clotting factors.
To restore normal blood flow as the vessel heals, the clot must eventually be removed. The process by which the clot is gradually degraded is called fibrinolysis. Like coagulation, fibrinolysis involves a fairly complicated series of protein catabolising reactions. During this process, the inactive protein plasminogen, released by endothelial cells around the site of blood vessel damage, is converted into the active enzyme plasmin, which gradually breaks down the fibrin of the clot. Additionally, bradykinin, a vasodilator, released from damaged tissues as a pain signal, reverses the effects of the serotonin and prostaglandins secreted by the platelets. This allows the smooth muscle in the walls of the vessels to relax and helps to restore the circulation.
Plasma Anticoagulants
An anticoagulant is any substance that opposes coagulation. Several circulating plasma anticoagulants play a role in limiting the coagulation process to the region of injury and restoring a normal, clot-free condition of blood. For instance, a cluster of proteins collectively referred to as the protein C system inactivates clotting factors involved in the intrinsic pathway. TFPI (tissue factor pathway inhibitor) inhibits the conversion of the inactive factor VII to the active form in the extrinsic pathway. Antithrombin inactivates factor X and opposes the conversion of prothrombin (factor II) to thrombin in the common pathway. And as noted earlier, basophils release heparin, a short-acting anticoagulant that also opposes prothrombin. Heparin is also found on the surfaces of cells lining the blood vessels. A pharmaceutical form of heparin is often administered therapeutically, for example, in surgical patients at risk for blood clots.
Disorders of Clotting
Either an insufficient or an excessive production of platelets can lead to severe bleeding on the hand or excessive platelet plug formation on the other. Both of these situations can be life-threatening. As discussed earlier, an insufficient number of platelets, called thrombocytopenia, typically results in the inability of blood to form clots. This can lead to excessive bleeding, even from minor wounds.
Another reason for failure of the blood to clot is the inadequate production of functional amounts of one or more clotting factors. This is the case in the genetic disorder haemophilia, which is actually a group of related disorders, the most common of which is haemophilia A, accounting for approximately 80 percent of cases. This disorder results in the inability to synthesise enough factor VIII. Haemophilia B is the second most common form, accounting for approximately 20 percent of cases. In this case, there is a deficiency of factor IX. Both of these defects are linked to the X chromosome and are typically passed from a healthy (carrier) mother to her male offspring, since males are XY. Females would need to inherit a defective gene from each parent to manifest the disease, since they are XX. Patients with haemophilia bleed from even minor internal and external wounds, and leak blood into joint spaces after exercise and into urine and stool. Haemophilia C is a rare condition that is triggered by an autosomal (not sex) chromosome defect that renders factor XI non-functional. It is not a true recessive condition, since even individuals with a single copy of the mutant gene show a tendency to bleed. Regular infusions of clotting factors isolated from healthy donors, or manufactured as recombinant proteins, can help prevent bleeding in haemophiliac patients. At some point, genetic therapy will become a viable option.
In contrast to the disorders characterised by thrombocytopenia is thrombocytosis, also mentioned earlier, a condition characterised by excessive numbers of platelets that increases the risk for excessive clot formation, a condition known as thrombosis. Inherited or acquired failures in the control or regulation of coagulation can also result in a tendency towards thrombosis: a condition referred to as thrombophilia or hypercoagulation. Deficiencies of protein C or antithrombin, for example, can predispose an individual to thrombosis. Acquired forms of thrombophilia include the autoimmune disease lupus, immune reactions to heparin, polycythaemia vera, sickle cell disease, pregnancy, and even obesity. A thrombus (plural = thrombi) is an aggregation of platelets, erythrocytes, and even WBCs typically trapped within a mass of fibrin strands. While the formation of a clot is normal following the haemostatic response to blood vessel damage just described, thrombi can form within an intact or only slightly damaged blood vessel. In a large vessel, a thrombus will adhere to the vessel wall and decrease the flow of blood and is referred to as a mural thrombus. In a small vessel, it may totally block the flow of blood and is termed an occlusive thrombus. Thrombi are most caused by vessel damage to the endothelial lining, which activates the clotting mechanism. These may include venous stasis, when blood in the veins, particularly in the legs, remains stationary for long periods. This is one of the dangers of long airplane flights in crowded conditions and may lead to deep vein thrombosis or atherosclerosis, an accumulation of fats, macrophages and cell debris in the walls of arteries. A thrombus can seriously impede blood flow to or from a region and will cause a local increase in blood pressure. If flow is to be maintained, the heart will need to generate a greater pressure to overcome the resistance.
When a portion of a thrombus breaks free from the vessel wall and enters the circulation, it is referred to as an embolus. An embolus that is carried through the bloodstream can be large enough to block a vessel critical to a major organ. When it becomes trapped, an embolus is called an embolism. In the heart, brain, or lungs, an embolism may accordingly cause a heart attack, a stroke, or a pulmonary embolism. These are medical emergencies.
Among the many known biochemical activities of aspirin is its role as an anticoagulant. Aspirin (acetylsalicylic acid) is very effective at inhibiting the aggregation of platelets. Physicians sometimes recommend that patients at risk for cardiovascular disease, which can lead to heart attack or stroke, take a low dose of aspirin on a daily basis as a preventive measure. However, aspirin can also lead to serious side effects, including increasing the risk of gastrointestinal ulcers. A patient is well advised to consult a physician before beginning any aspirin regimen.
A class of drugs collectively known as thrombolytic agents can help speed up the degradation of an abnormal clot. If a thrombolytic agent is administered to a patient within 3 hours following a thrombotic stroke, the patient’s prognosis improves significantly. However, some strokes are not caused by thrombi, but by haemorrhage. Thus, the cause must be determined before treatment begins. Tissue plasminogen activator is an enzyme that catalyses the conversion of plasminogen to plasmin, the primary enzyme that breaks down clots. It is released naturally by endothelial cells but is also used in clinical medicine. New research is progressing using compounds isolated from the venom of some species of snakes, particularly vipers and cobras, which may eventually have therapeutic value as thrombolytic agents. Streptokinase, an enzyme produced by some species of streptococci, has long been used to help degrade the thrombus in heart attack cases.
Section Review
Haemostasis is the physiological process by which bleeding ceases. Haemostasis involves three basic steps: vascular spasm, the formation of a platelet plug, and coagulation, by which clotting factors promote the formation of a fibrin clot. Fibrinolysis is the process in which a clot is degraded in a healing vessel. Anticoagulants are substances that oppose coagulation. They are important in limiting the extent and duration of clotting. Inadequate clotting can result from too few platelets, or inadequate production of clotting factors, for instance, in the genetic disorder haemophilia. Excessive clotting, called thrombosis, can be caused by excessive numbers of platelets or deficiencies in coagulation control factors. A thrombus is a collection of fibrin, platelets, and erythrocytes that has accumulated along the lining of a blood vessel, whereas an embolus is a thrombus that has broken free from the vessel wall and is circulating in the bloodstream.
Review Questions
Critical Thinking Questions
Click the drop down below to review the terms learned from this chapter.
