8.5 Transport of Gases
Learning Objectives
By the end of this section, you will be able to:
- Describe the principles of oxygen transport
- Describe the structure of haemoglobin
- Compare and contrast foetal and adult haemoglobin
- Describe the principles of carbon dioxide transport
The other major activity in the lungs is the process of respiration, the process of gas exchange. The function of respiration is to provide oxygen for use by body cells during cellular respiration and to eliminate carbon dioxide, a waste product of cellular respiration, from the body. In order for the exchange of oxygen and carbon dioxide to occur, both gases must be transported between the external and internal respiration sites. Although carbon dioxide is more soluble than oxygen in blood, both gases require a specialised transport system for the majority of the gas molecules to be moved between the lungs and other tissues.
Oxygen Transport in the Blood
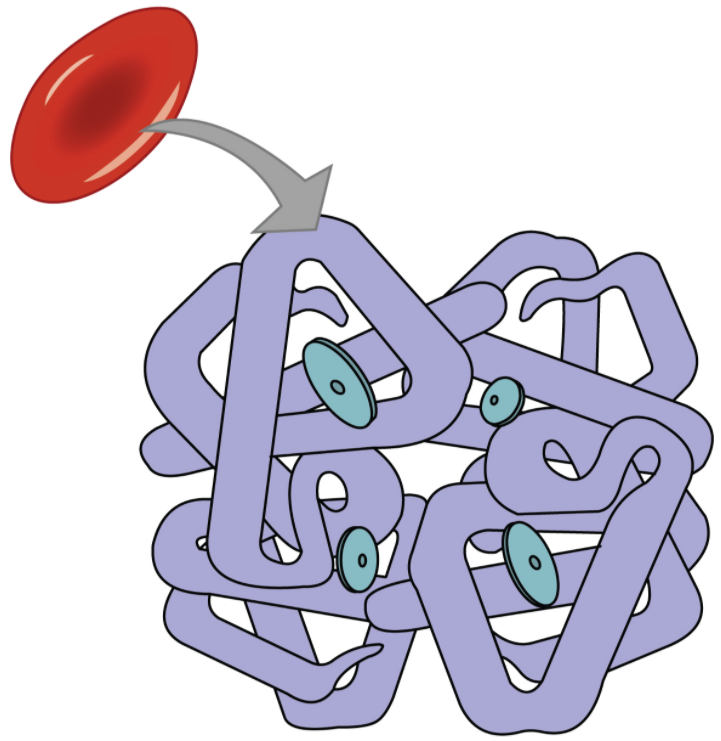
Even though oxygen is transported via the blood, you may recall that oxygen is not very soluble in liquids. A small amount of oxygen does dissolve in the blood and is transported in the bloodstream, but it is only about 1.5% of the total amount. The majority of oxygen molecules are carried from the lungs to the body’s tissues by a specialised transport system, which relies on the erythrocyte—the red blood cell. Erythrocytes contain a metalloprotein, haemoglobin, which serves to bind oxygen molecules to the erythrocyte (Figure 8.5.1). Haem is the portion of haemoglobin that contains iron, and it is haem that binds oxygen. One haemoglobin molecule contains iron-containing Haem molecules, and because of this, each haemoglobin molecule is capable of carrying up to four molecules of oxygen. As oxygen diffuses across the respiratory membrane from the alveolus to the capillary, it also diffuses into the red blood cell and is bound by haemoglobin. The following reversible chemical reaction describes the production of the final product, oxyhaemoglobin (Hb–O2), which is formed when oxygen binds to haemoglobin. Oxyhaemoglobin is a bright red-coloured molecule that contributes to the bright red colour of oxygenated blood.
Hb + O2 ↔ Hb − O2
In this formula, Hb represents reduced haemoglobin, that is, haemoglobin that does not have oxygen bound to it. There are multiple factors involved in how readily haem binds to and dissociates from oxygen, which will be discussed in the subsequent sections.
Function of Haemoglobin
Haemoglobin is composed of subunits, a protein structure that is referred to as a quaternary structure. Each of the four subunits that make up haemoglobin is arranged in a ring-like fashion, with an iron atom covalently bound to the haem in the centre of each subunit. Binding of the first oxygen molecule causes a conformational change in haemoglobin that allows the second molecule of oxygen to bind more readily. As each molecule of oxygen is bound, it further facilitates the binding of the next molecule, until all four haem sites are occupied by oxygen. The opposite occurs as well: After the first oxygen molecule dissociates and is “dropped off” at the tissues, the next oxygen molecule dissociates more readily. When all four haem sites are occupied, the haemoglobin is said to be saturated. When one to three haem sites are occupied, the haemoglobin is said to be partially saturated. Therefore, when considering the blood as a whole, the percent of the available haem units that are bound to oxygen at a given time is called haemoglobin saturation. Haemoglobin saturation of 100 percent means that every haem unit in all of the erythrocytes of the body is bound to oxygen. In a healthy individual with normal haemoglobin levels, haemoglobin saturation generally ranges from 95 percent to 99 percent.
Oxygen Dissociation from Haemoglobin
Partial pressure is an important aspect of the binding of oxygen to and disassociation from haem. An oxygen–haemoglobin dissociation curve is a graph that describes the relationship of partial pressure to the binding of oxygen to haem and its subsequent dissociation from haem (Figure 8.5.2). Remember that gases travel from an area of higher partial pressure to an area of lower partial pressure. In addition, the affinity of an oxygen molecule for haem increases as more oxygen molecules are bound. Therefore, in the oxygen–haemoglobin saturation curve, as the partial pressure of oxygen increases, a proportionately greater number of oxygen molecules are bound by haem. Not surprisingly, the oxygen–haemoglobin saturation/dissociation curve also shows that the lower the partial pressure of oxygen, the fewer oxygen molecules are bound to haem. As a result, the partial pressure of oxygen plays a major role in determining the degree of binding of oxygen to haem at the site of the respiratory membrane, as well as the degree of dissociation of oxygen from haem at the site of body tissues.
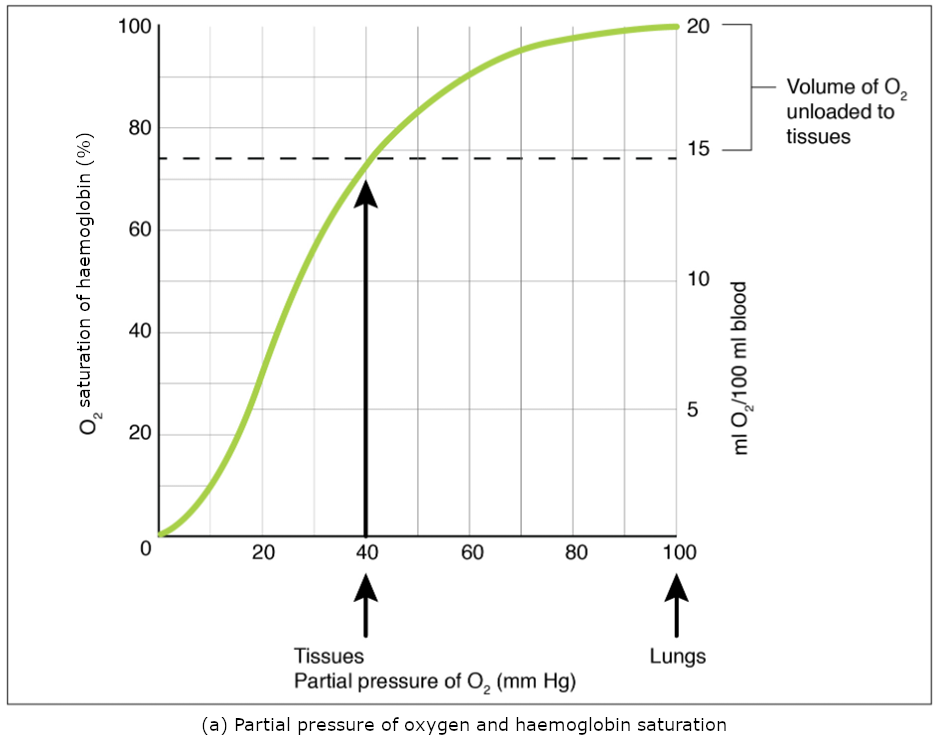
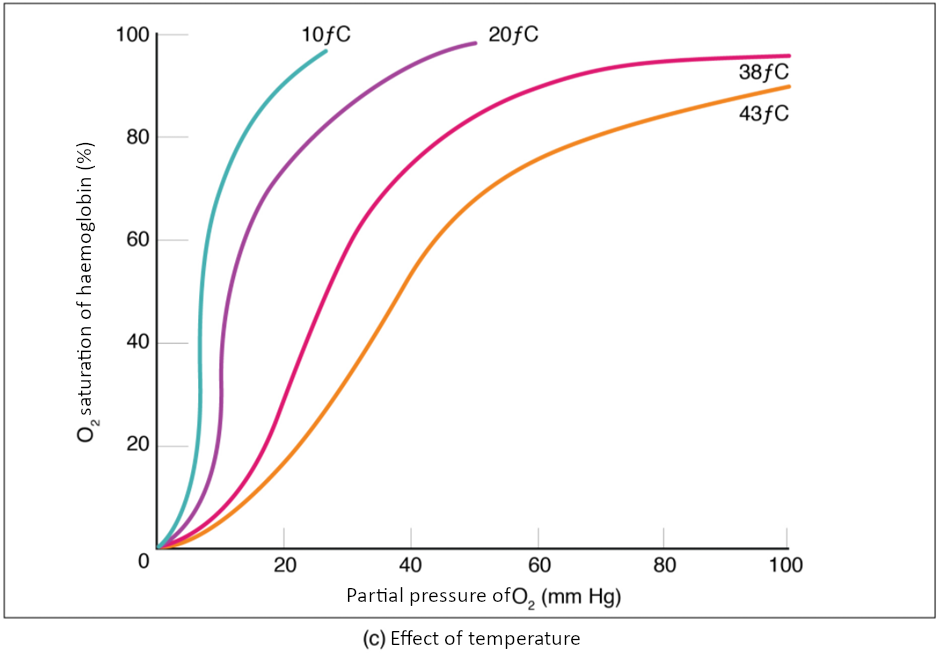
The mechanisms behind the oxygen–haemoglobin saturation/dissociation curve also serve as automatic control mechanisms that regulate how much oxygen is delivered to different tissues throughout the body. This is important because some tissues have a higher metabolic rate than others. Highly active tissues, such as muscle, rapidly use oxygen to produce ATP, lowering the partial pressure of oxygen in the tissue to about 20 mm Hg. The partial pressure of oxygen inside capillaries is about 100 mm Hg, so the difference between the two becomes quite high, about 80 mm Hg. As a result, a greater number of oxygen molecules dissociate from haemoglobin and enter the tissues. The reverse is true of tissues, such as adipose (body fat), which have lower metabolic rates. Because less oxygen is used by these cells, the partial pressure of oxygen within such tissues remains relatively high, resulting in fewer oxygen molecules dissociating from haemoglobin and entering the tissue interstitial fluid. Although venous blood is said to be deoxygenated, some oxygen is still bound to haemoglobin in its red blood cells. This provides an oxygen reserve that can be used when tissues suddenly demand more oxygen.
Factors other than partial pressure also affect the oxygen–haemoglobin saturation/dissociation curve. For example, a higher temperature promotes haemoglobin and oxygen to dissociate faster, whereas a lower temperature inhibits dissociation (see Figure 8.5.2, middle). However, the human body tightly regulates temperature, so this factor may not affect gas exchange throughout the body. The exception to this is in highly active tissues, which may release a larger amount of energy than is given off as heat. As a result, oxygen readily dissociates from haemoglobin, which is a mechanism that helps to provide active tissues with more oxygen.
Certain hormones, such as androgens, adrenaline, thyroid hormones, and growth hormone, can affect the oxygen–haemoglobin saturation/dissociation curve by stimulating the production of a compound called 2,3-bisphosphoglycerate (BPG) by erythrocytes. BPG is a by-product of glycolysis. Because erythrocytes do not contain mitochondria, glycolysis is the sole method by which these cells produce ATP. BPG promotes the dissociation of oxygen from haemoglobin. Therefore, the greater the concentration of BPG, the more readily oxygen dissociates from haemoglobin, despite its partial pressure.
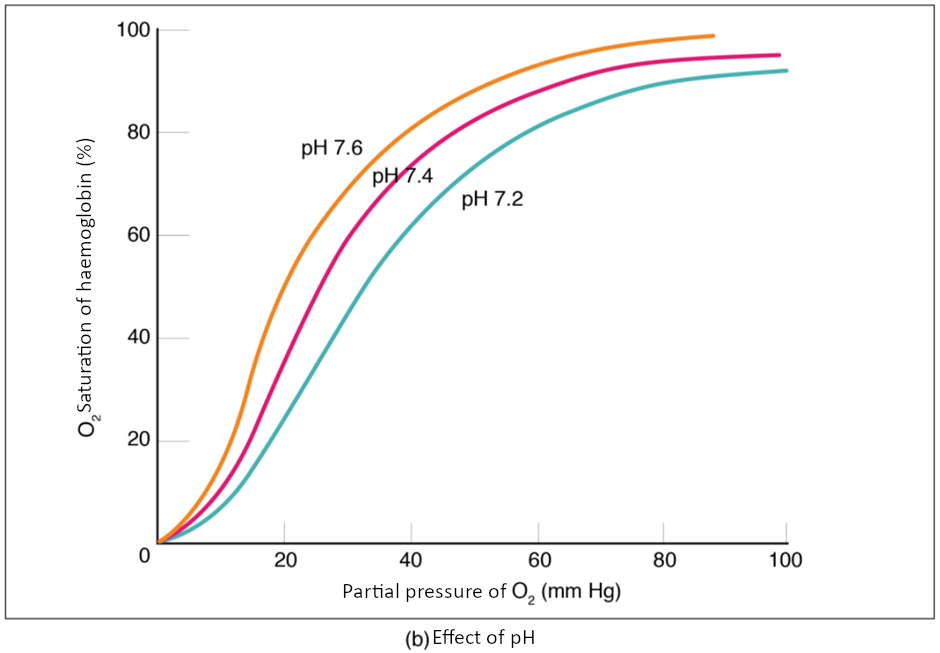
The pH of the blood is another factor that influences the oxygen–haemoglobin saturation/dissociation curve (see Figure 8.5.2). The Bohr effect is a phenomenon that arises from the relationship between pH and oxygen’s affinity for haemoglobin: A lower, more acidic pH promotes oxygen dissociation from haemoglobin. In contrast, a higher, or more basic, pH inhibits oxygen dissociation from haemoglobin. The greater the amount of carbon dioxide in the blood, the more molecules that must be converted, which in turn generates hydrogen ions and thus lowers blood pH. Furthermore, blood pH may become more acidic when certain by-products of cell metabolism, such as lactic acid, carbonic acid, and carbon dioxide, are released into the bloodstream.
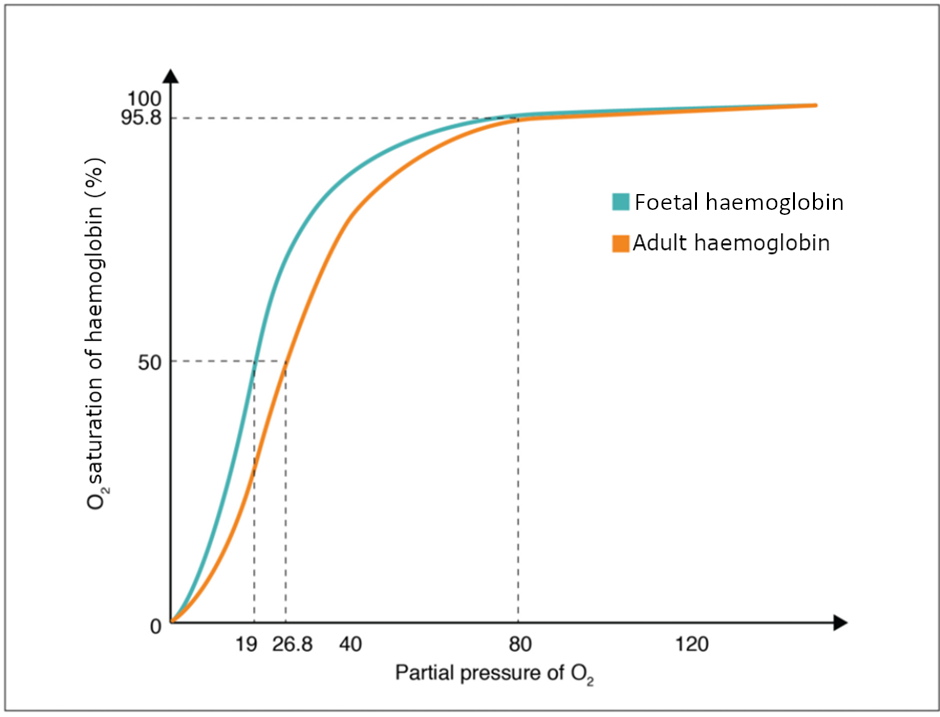
Haemoglobin of the Foetus
The foetus has its own circulation with its own erythrocytes; however, it is dependent on the mother for oxygen. Blood is supplied to the foetus by way of the umbilical cord, which is connected to the placenta and separated from maternal blood by the chorion. The mechanism of gas exchange at the chorion is similar to gas exchange at the respiratory membrane. However, the partial pressure of oxygen is lower in the maternal blood in the placenta, at about 35 to 50 mm Hg, than it is in maternal arterial blood. The difference in partial pressures between maternal and foetal blood is not large, as the partial pressure of oxygen in foetal blood at the placenta is about 20 mm Hg. Therefore, there is not as much diffusion of oxygen into the foetal blood supply. The foetus’ haemoglobin overcomes this problem by having a greater affinity for oxygen than maternal haemoglobin (Figure 8.5.3). Both foetal and adult haemoglobin have four subunits, but two of the subunits of foetal haemoglobin have a different structure that causes foetal haemoglobin to have a greater affinity for oxygen than does adult haemoglobin.
Carbon Dioxide Transport in the Blood
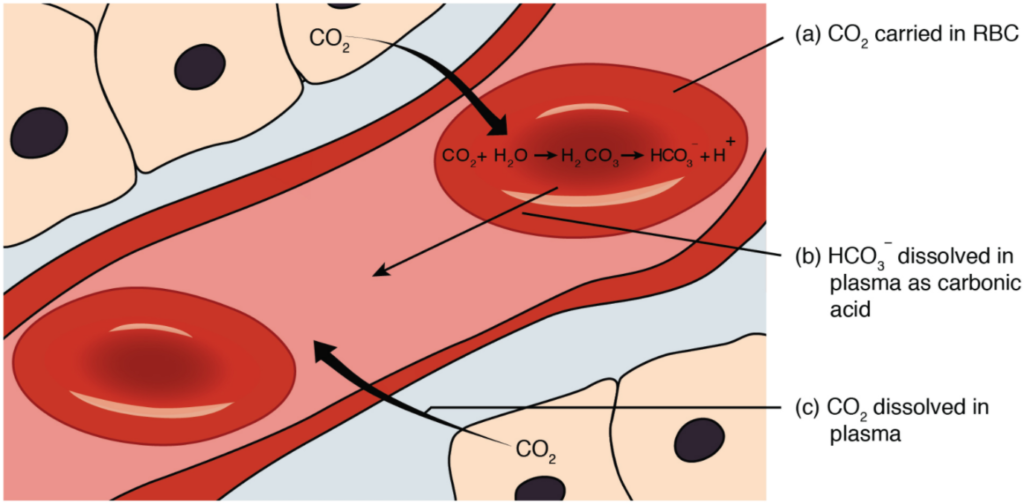
Carbon dioxide is transported by three major mechanisms. The first mechanism of carbon dioxide transport is by blood plasma, as some carbon dioxide molecules dissolve in the blood. The second mechanism is transport in the form of bicarbonate (HCO3–), which also dissolves in plasma. The third mechanism of carbon dioxide transport is similar to the transport of oxygen by erythrocytes (Figure 8.5.4).
Dissolved Carbon Dioxide
Although carbon dioxide is not considered to be highly soluble in blood, a small fraction—about 7 to 10 percent—of the carbon dioxide that diffuses into the blood from the tissues dissolves in plasma. The dissolved carbon dioxide then travels in the bloodstream and when the blood reaches the pulmonary capillaries, the dissolved carbon dioxide diffuses across the respiratory membrane into the alveoli, where it is then exhaled during pulmonary ventilation.
Bicarbonate Buffer
A large fraction—about 70 percent—of the carbon dioxide molecules that diffuse into the blood is transported to the lungs as bicarbonate. Most bicarbonate is produced in erythrocytes after carbon dioxide diffuses into the capillaries, and subsequently into red blood cells. Carbonic anhydrase (CA) causes carbon dioxide and water to form carbonic acid (H2CO3), which dissociates into two ions: bicarbonate (HCO3–) and hydrogen (H+). The following formula depicts this reaction:
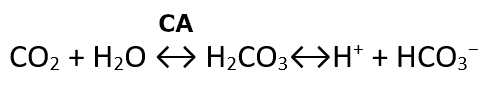
Bicarbonate tends to build up in the erythrocytes, so that there is a greater concentration of bicarbonate in the erythrocytes than in the surrounding blood plasma. As a result, some of the bicarbonate will leave the erythrocytes and move down its concentration gradient into the plasma in exchange for chloride (Cl–) ions. This phenomenon is referred to as the chloride shift and occurs because by exchanging one negative ion for another negative ion, neither the electrical charge of the erythrocytes nor that of the blood is altered.
At the pulmonary capillaries, the chemical reaction that produced bicarbonate (shown above) is reversed, and carbon dioxide and water are the products. Much of the bicarbonate in the plasma re-enters the erythrocytes in exchange for chloride ions. Hydrogen ions and bicarbonate ions join to form carbonic acid, which is converted into carbon dioxide and water by carbonic anhydrase. Carbon dioxide diffuses out of the erythrocytes and into the plasma, where it can further diffuse across the respiratory membrane into the alveoli to be exhaled during pulmonary ventilation.
Carbaminohaemoglobin
About 20 percent of carbon dioxide is bound by haemoglobin and is transported to the lungs. Carbon dioxide does not bind to iron as oxygen does; instead, carbon dioxide binds amino acid moieties on the globin portions of haemoglobin to form carbaminohaemoglobin, which forms when haemoglobin and carbon dioxide bind. When haemoglobin is not transporting oxygen, it tends to have a bluish-purple tone to it, creating the darker maroon colour typical of deoxygenated blood. The following formula depicts this reversible reaction:
CO2 + Hb ↔ HbCO2
Similar to the transport of oxygen by haem, the binding and dissociation of carbon dioxide to and from haemoglobin is dependent on the partial pressure of carbon dioxide. Because carbon dioxide is released from the lungs, blood that leaves the lungs and reaches body tissues has a lower partial pressure of carbon dioxide than is found in the tissues. As a result, carbon dioxide leaves the tissues because of its higher partial pressure, enters the blood, and then moves into red blood cells, binding to haemoglobin. In contrast, in the pulmonary capillaries, the partial pressure of carbon dioxide is high compared to within the alveoli. As a result, carbon dioxide dissociates readily from haemoglobin and diffuses across the respiratory membrane into the air.
In addition to the partial pressure of carbon dioxide, the oxygen saturation of haemoglobin and the partial pressure of oxygen in the blood also influence the affinity of haemoglobin for carbon dioxide. The Haldane effect is a phenomenon that arises from the relationship between the partial pressure of oxygen and the affinity of haemoglobin for carbon dioxide. haemoglobin that is saturated with oxygen does not readily bind carbon dioxide. However, when oxygen is not bound to haem and the partial pressure of oxygen is low, haemoglobin readily binds to carbon dioxide.
Section Review
Oxygen is primarily transported through the blood by erythrocytes. These cells contain a metalloprotein called haemoglobin, which is composed of four subunits with a ring-like structure. Each subunit contains one atom of iron bound to a molecule of haem. Haem binds oxygen so that each haemoglobin molecule can bind up to four oxygen molecules. When all the haem units in the blood are bound to oxygen, haemoglobin is considered to be saturated. Haemoglobin is partially saturated when only some haem units are bound to oxygen. An oxygen–haemoglobin saturation/dissociation curve is a common way to depict the relationship of how easily oxygen binds to or dissociates from haemoglobin as a function of the partial pressure of oxygen. As the partial pressure of oxygen increases, the more readily haemoglobin binds to oxygen. At the same time, once one molecule of oxygen is bound by haemoglobin, additional oxygen molecules more readily bind to haemoglobin. Other factors such as temperature, pH, the partial pressure of carbon dioxide, and the concentration of 2,3-bisphosphoglycerate can enhance or inhibit the binding of haemoglobin and oxygen as well. Foetal haemoglobin has a different structure than adult haemoglobin, which results in foetal haemoglobin having a greater affinity for oxygen than adult haemoglobin.
Carbon dioxide is transported in blood by three different mechanisms: as dissolved carbon dioxide, as bicarbonate, or as carbaminohaemoglobin. A small portion of carbon dioxide remains. The largest amount of transported carbon dioxide is as bicarbonate, formed in erythrocytes. For this conversion, carbon dioxide is combined with water with the aid of an enzyme called carbonic anhydrase. This combination forms carbonic acid, which spontaneously dissociates into bicarbonate and hydrogen ions. As bicarbonate builds up in erythrocytes, it is moved across the membrane into the plasma in exchange for chloride ions by a mechanism called the chloride shift. At the pulmonary capillaries, bicarbonate re-enters erythrocytes in exchange for chloride ions, and the reaction with carbonic anhydrase is reversed, recreating carbon dioxide and water. Carbon dioxide then diffuses out of the erythrocyte and across the respiratory membrane into the air. An intermediate amount of carbon dioxide binds directly to haemoglobin to form carbaminohaemoglobin. The partial pressures of carbon dioxide and oxygen, as well as the oxygen saturation of haemoglobin, influence how readily haemoglobin binds carbon dioxide. The less saturated haemoglobin is and the lower the partial pressure of oxygen in the blood is, the more readily haemoglobin binds to carbon dioxide. This is an example of the Haldane effect.
Review Questions
Critical Thinking Questions
Click the drop down below to review the terms learned from this chapter.